Answer: Option (c) is the correct answer.
Step-by-step explanation:
As the given reaction equation is as follows.
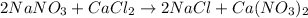
The coefficient present adjacent to the chemical formula of a compound or molecule represents the atoms of each element present in the formula or reaction.
Since, no coefficient is present in front of
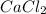 and
and
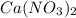 on either side of the reaction mixture. Hence, it means only one atom of calcium is present on either side of the reaction mixture.
on either side of the reaction mixture. Hence, it means only one atom of calcium is present on either side of the reaction mixture.
Thus, we can conclude that one atom of calcium (Ca) are there on either side of the equation.