First we need to write a balance equation for this redox reaction
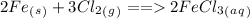
In the above reaction
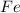 on reactant side has oxidation state 0, while on the product side it has an oxidation state of +3. On the other hand,
on reactant side has oxidation state 0, while on the product side it has an oxidation state of +3. On the other hand,
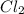 has oxidation state of 0 on reactant side and an oxidation state of -1 on product side.
has oxidation state of 0 on reactant side and an oxidation state of -1 on product side.
The metal
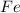 loses electrons to form
loses electrons to form
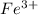 ion and is oxidized. So we write an oxidation half reaction.
ion and is oxidized. So we write an oxidation half reaction.
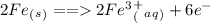
and the
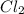 molecule gains electrons to form
molecule gains electrons to form
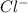 ions and is reduced. The reduction half reaction is
ions and is reduced. The reduction half reaction is
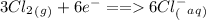 .
.