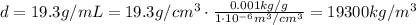Answer: 19.3 g/mL, or 19,300 kg/m^3
Step-by-step explanation:
The density of the piece of metal is given by:
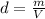
where
m = 25.1 g is the mass of the piece of metal
V = 1.3 mL is the volume
Substituting the numbers into the formula, we find
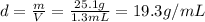
If we want to convert into SI units: