Answer:
1.4 atm
Step-by-step explanation:
According to Gay Lussac’s law, the pressure of gas is inversely proportional t its temperature, when the volume is constant
Initial temperature = 125 + 273 = 398 K
Final temperature = 182 + 273 = 455 K
Initial pressure = 1.22 atm
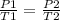
1.22 atm / 398 K = P₂/ 455 K
P₂ =
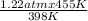 = 1.4 atm
= 1.4 atm