Answer: The name of the compound formed will be magnesium iodide.
Step-by-step explanation:
An ionic bond is formed by the complete transfer of electrons from one element to another element. An atom which looses electrons is known as electropositive element and is generally metal. The atom which gains electrons is known as electronegative element and is generally non-metal.
We are given:
Charge on iodine (non-metal) atom = -1
Charge on magnesium (metal) atom = +2
By criss-cross method, in which the charges of the atom gets exchanged to form the subscripts of the atoms. So, the chemical formula for the compound formed will be
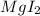
The nomenclature of ionic compounds is given by:
- Positive ion is written first.
- The negative ion is written next and a suffix is added at the end of the negative ion. The suffix written is '-ide'.
Hence, the name of
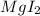 is magnesium iodide.
is magnesium iodide.