Answer:
The pH of the solution is 3.
Step-by-step explanation:
Moles of HCl = 0.0020 mol
Volume of the solution = 2000 mL = 2 L
1 L = 1000 mL
Molarity =
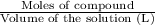

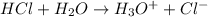
1 mol of HCl forms 1 mole of hydronium ion and 1 mol of chloride ions.
Then 0.0010 M of of HCl forms 0.0010 M of hydronium ion
The molarity of hydronium ion = 0.0010 M
The pH of the solution is defined as negative logarithm of hydronium ion or hydrogen ion concentration in a solution.
![pH=-\log[H_3O^+]](https://img.qammunity.org/2019/formulas/chemistry/high-school/1xyl8t62rgf63wq4a9vmmmzn73ufb97fcz.png)
![pH=-\log[0.0010 M]=3](https://img.qammunity.org/2019/formulas/chemistry/high-school/36969zvn66enejbmq75ptqp7jhj78yviq3.png)
The pH of the solution is 3.