Answer : The hydronium ion concentration of a solution is
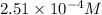 .
.
Solution : Given,
pH = 3.60
pH : It is defined as the negative logarithm of hydronium ion concentration.
Formula used :
![pH=-log[H_3O^+]](https://img.qammunity.org/2019/formulas/chemistry/middle-school/t1qqw59wbrmyd1orvr9wwtomxygflv3319.png)
Now put all the values in this formula, we get
![3.60=-log[H_3O^+]\\](https://img.qammunity.org/2019/formulas/chemistry/middle-school/2xu5jfmtlta85zc7ks2o1w36q97sqvu7qf.png)
![[H_3O^+]=antilog(-3.60)](https://img.qammunity.org/2019/formulas/chemistry/middle-school/rkgwcdc480a106j66pxl1lpqs0vvdtznxw.png)
![[H_3O^+]=0.000251=2.50* 10^(-4)](https://img.qammunity.org/2019/formulas/chemistry/middle-school/7lfzk4kv59275lw7ne849h2ydojjycar8f.png)
Therefore, the hydronium ion concentration of a solution is
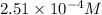 .
.