Answer:- Volume decreases by a factor of 1.15.
Solution:- At constant pressure, volume of the gas is directly proportional to the the kelvin temperature.
The equation is written as:
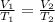
where,
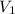 is the volume at initial temperature
is the volume at initial temperature
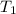 and
and
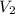 is the volume at final temperature
is the volume at final temperature
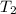 .
.
Temperature must be in kelvins. So, let's convert both the temperatures to kelvin.
To convert degree C to kelvin we add 273.
So,
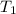 = 100 + 273 = 373 K
= 100 + 273 = 373 K
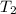 = 50 + 273 = 323 K
= 50 + 273 = 323 K
The equation could also be written as:-
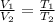
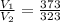
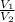 = 1.15
= 1.15
From here we could say that the volume decreases by a factor of 1.15.
For example if the initial volume
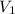 is 1 L then final volume
is 1 L then final volume
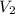 will be
will be
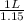 that is 0.87 L.
that is 0.87 L.