Answer: Option (B) is the correct answer.
Step-by-step explanation:
According to the IUPAC nomenclature when a metal combines with a non-metal then name of cation remains the same. Whereas suffixe "-ide" is added at the end of the name of anion.
For example, in case of
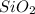 the cation is silicon and anion is oxygen. As two atoms of oxygen are involved in this compound so, the prefix "-di" is added before the name of anion.
the cation is silicon and anion is oxygen. As two atoms of oxygen are involved in this compound so, the prefix "-di" is added before the name of anion.
Hence, we can conclude that the name of
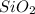 is silicon dioxide.
is silicon dioxide.