Answer:- 121 kPa.
Solution:- mass, temperature and volume are given for carbon dioxide gas asked to calculate the pressure this gas exerts on the container.
It is based on ideal gas law equation, PV = nRT .
Where, P is the pressure in atm, V is the volume in Liters, n is the number of moles of the gas, R is the universal gas constant and it's value is
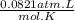 and T is the kelvin temperature.
and T is the kelvin temperature.
Given data:- mass = 88.0 g
T = 291 K
V = 40.0 L
P = ?
We need to convert the mass to moles and for this we divide the mass by molar mass. Molar mass of carbon dioxide gas is 44.01 gram per mol.
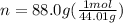
n = 2.00 mol
The equation could be rearranged for pressure as:
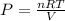
Let's plug in the values and solve this for P.
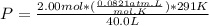
P = 1.1946 atm
Since the answer is asked to report in kPa, let's convert atm to kPa.
1 atm = 101.325 kPa
So,
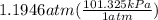
= 121 kPa
So, the pressure this gas exerts on the container is 121 kPa.