Answer:- a) Two molecules should decompose to form the given products.
Explanations:- The given equation is:
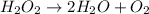
A balanced equation must have equal number of atoms of each element. If we look at the given reaction then hydrogen peroxide is decomposing to give water and oxygen gas. Also, if we look at the H and O then left side has two H and two O where as the right side has four H and four O.
Neither H nor O is balanced. Left side has exactly half H and O as compared to the right side and so to balance this we need to multiply hydrogen peroxide by two.
The balanced equation will be:
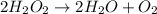
Hence, choice a is correct. "Two
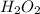 molecules should decompose to form the given products."
molecules should decompose to form the given products."