According to Bronsted-Lowry theory of acids and bases, an acid is proton donor and a base is proton acceptor. When an acid loses the proton, it forms the conjugate base of the acid. Similarly when a base accepts a proton, it forms the conjugate acid if that base. A conjugate acid base pair differs by a single proton.
Given the weak acid
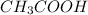 . It can lose a proton to form the conjugate base.
. It can lose a proton to form the conjugate base.
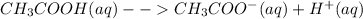
Therefore, the conjugate base of
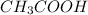 is
is
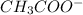 .
.