The question is incomplete, here is the complete question:
Which type of atom is expected in the reactants of this chemical reaction?
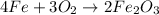
A. Iron (Fe)
B. Gold (Au)
C. Nitrogen (N)
D. Chlorine (Cl)
Answer: The atom which is expected as the reactant in the given chemical reaction is iron (Fe)
Step-by-step explanation:
In a chemical equation, the chemical species are termed as reactants or products.
- Reactants are defined as the species which react in the reaction and are written on the left side of the reaction arrow.
- Products are defined as the species which are produced in the reaction and are written on the right side of the reaction arrow.
For the given chemical reaction:
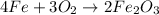
The reactants to the given chemical reaction are iron (Fe) metal and oxygen gas
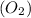
The products to the given chemical reaction is iron (III) oxide
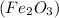
Hence, the atom which is expected as the reactant in the given chemical reaction is iron (Fe)