Answer: Option (B) is the correct answer.
Step-by-step explanation:
pH is defined as the measurement of hydrogen or hydroxide ions in a solution.
Mathematically, pH =
![-log[H^(+)]](https://img.qammunity.org/2019/formulas/chemistry/college/guink5px3a3s9xbc3r41v2pyg7w9jvymq8.png)
It is given that concentration of
![[H^(+)]](https://img.qammunity.org/2019/formulas/chemistry/college/75vk4lm3dg3i0qv728qhnrrb5qib1loutc.png) or hydronium ion
or hydronium ion
![[H_(3)O^(+)]](https://img.qammunity.org/2019/formulas/chemistry/high-school/z87fpn7kestotksu3rnc4344ei1g9j0mtz.png) is
is
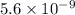 .
.
Therefore, calculate the pH as follows.
pH =
![-log[H^(+)]](https://img.qammunity.org/2019/formulas/chemistry/college/guink5px3a3s9xbc3r41v2pyg7w9jvymq8.png)
=
![-log[5.6 * 10^(-9)]](https://img.qammunity.org/2019/formulas/chemistry/college/vwzef46bn6nrzgkah8ecov9uayca197sak.png)
= 8.25
Thus, we can conclude pH of the solution is 8.25.