The correct answer is B.
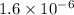
Explanation
The question provides us with information about the concentration of
![[OH^(-) ]](https://img.qammunity.org/2019/formulas/chemistry/college/f77wuw8stzpxsh7fluujzwruo3bzkvyjgv.png) and we are supposed to use this information to find the
and we are supposed to use this information to find the
![[H^(+) ]](https://img.qammunity.org/2019/formulas/chemistry/college/s4zi1q3jsr7wemoju3anplmn4i8mt6f60x.png) concentration. The pH of a solution gives us information about
concentration. The pH of a solution gives us information about
![[H^(+) ]](https://img.qammunity.org/2019/formulas/chemistry/college/s4zi1q3jsr7wemoju3anplmn4i8mt6f60x.png)
If we the reaction is in equilibrium and with standard conditions of 25°C, then pH is given by the formula
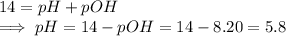 .
.
Once we find the pH we use the relationship
![pH= -log[H^(+) ]](https://img.qammunity.org/2019/formulas/chemistry/college/oeojbwjfau4hs7qf1v9ly3g032vdimauii.png) , then the
, then the
![[H^(+) ]](https://img.qammunity.org/2019/formulas/chemistry/college/s4zi1q3jsr7wemoju3anplmn4i8mt6f60x.png) ,is given by
,is given by
![pH = -log[H^(+) ]\\\implies [H^(+) ]= 10^(-pH) = 10^(-5.8)= 1.58 * 10^(-6)](https://img.qammunity.org/2019/formulas/chemistry/college/y79qpk2nxbxxpoby2x0d62qas93uwj74lo.png)