Answer : 5.83 grams of Zinc metal are required to produce the given amount of H2.
Explanation :
The chemical equation for the reaction between zinc metal and hydrochloric acid is given below.

Step 1 : Find moles of H2.
Let us find out the moles of H2 gas produced at STP.
One mole of any gas occupies 22.4 L at STP.
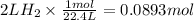
Step 2 : Find moles of Zn.
The mole ratio of Zn and H2 is 1 : 1 .
Therefore the moles of Zn required to produce the given amount of H2 are 0.0893 mol.
Step 3 : Convert mol Zn to grams.
Convert the above moles to grams using molar mass of Zn.
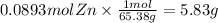
5.83 grams of Zinc metal are required to produce the given amount of H2.