If molecules are in a closed container then we expect the pressure to increase as the kinetic energy increases. This is because the atoms of an element collide with the walls of the container and increase the pressure.
If we use the formula
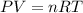 , where P is the pressure, V is the volume, n is the number of moles, R the ideal gas constant and T is the temperature. According to the formula, P is directly proportional to temperature. An increase in temperature leads to an increase in pressure.
, where P is the pressure, V is the volume, n is the number of moles, R the ideal gas constant and T is the temperature. According to the formula, P is directly proportional to temperature. An increase in temperature leads to an increase in pressure.
Since we know that temperature is the average kinetic energy of molecules present. It means as we increase the temperature we increase the kinetic energy of the molecules which in turn leads to an increase in the pressure.