The correct statements are "Each orbit holds a fixed number of electrons" and "The n=1 orbit can only hold two electrons." According to the Bohr model, the maximum number of electrons that can occupy an orbit is given by
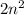 , where n is the number of the orbit. For instance, when n=1 it means
, where n is the number of the orbit. For instance, when n=1 it means
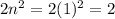 . This particular orbit can only hold up to two electrons. Even though the electrons can gain energy and move to higher orbits or electrons from higher orbits can lose energy and drop to the n=1 level, the energy level would not allow more electrons to enter the orbit once it is full. Again the octet rule, which states that atoms achieve stability by having 8 valence electrons, limits the maximum number of electrons that can be occupied by an orbit. The gain and loss of electrons is done to achieve the noble gas configuration and once that is reached no more electron can be added to an orbit
. This particular orbit can only hold up to two electrons. Even though the electrons can gain energy and move to higher orbits or electrons from higher orbits can lose energy and drop to the n=1 level, the energy level would not allow more electrons to enter the orbit once it is full. Again the octet rule, which states that atoms achieve stability by having 8 valence electrons, limits the maximum number of electrons that can be occupied by an orbit. The gain and loss of electrons is done to achieve the noble gas configuration and once that is reached no more electron can be added to an orbit