Answer: The correct option is A.
Explanation: To calculate the volume at the end , we use Charles's Law, which states that the volume of the gas is directly proportional to the temperature of the gas at constant pressure.
Mathematically,
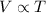
or
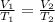




Putting all the values in above equation, we get

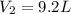
Hence, the correct option is A.