Answer: The correct statements are all three elements are part of the boron family and all three elements tend to form
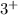 cations when reacting with metals.
cations when reacting with metals.
Step-by-step explanation:
We are given three elements Boron (B), Aluminium (Al) and Gallium (Ga).
All the elements written above belong to Group 13 and this group elements are known as Boron family.
The valence electronic configuration of the elements belonging to this group is
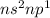 where 'n' is the period number.
where 'n' is the period number.
The number of valence electrons of these elements are 3.
These elements will loose 3 electrons in order to attain stable electronic configuration. Thus, after loosing 3 electrons, they will form +3 cations.
From the given elements, only boron forms covalent compounds. Rest all the elements forms ionic compounds.
Hence, the correct statements are all three elements are part of the boron family and all three elements tend to form
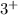 cations when reacting with metals.
cations when reacting with metals.