Answer : The mass of sulfuric acid needed is
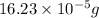 .
.
Solution : Given,
pH = 8.94
Volume of solution = 380 ml =
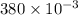
Molar mass of sulfuric acid = 98.079 g/mole
As we know,
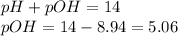
![pOH=-log[OH^-]](https://img.qammunity.org/2019/formulas/chemistry/middle-school/bb6nd3dwmelw6hrf97fgv6xj1ptgdgg61f.png)
![5.06=-log[OH^-]](https://img.qammunity.org/2019/formulas/chemistry/middle-school/329488zmdk6itgiu0vmfu722wfndisidhy.png)
![[OH^-]=0.00000871=8.71* 10^(-6)mole/L](https://img.qammunity.org/2019/formulas/chemistry/middle-school/8s9c370ofrnbc4mcum8vok6yn08bjpwsad.png)
Now we have to calculate the moles of
 .
.
Formula used :
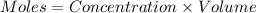
![\text{ Moles of }[OH^-]=\text{ Concentration of }[OH^-]* Volume\\\text{ Moles of }[OH^-]=(8.71* 10^(-6)mole/L)* (380* 10^(-3)L)=3309.8* 10^(-9)moles](https://img.qammunity.org/2019/formulas/chemistry/middle-school/g28i11pmfic03u81m66e47mifjl2tepnqz.png)
For neutralization, equal number of moles of
 ions will neutralize same number of
ions will neutralize same number of
 ions.
ions.
![\text{ Moles of }[OH^-]=\text{ Moles of }[H^+]=3309.8* 10^(-9)moles](https://img.qammunity.org/2019/formulas/chemistry/middle-school/xwu9mq1rqaezjzu6jyqx9ih6folj8avnv6.png)
As,
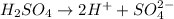
From this reaction, we conclude that
2 moles of
 ion is given by the 1 mole of
ion is given by the 1 mole of

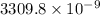 moles of
moles of
 ion is given by
ion is given by
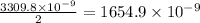 moles of
moles of

Now we have to calculate the mass of sulfuric acid.
Mass of sulfuric acid = Moles of
 × Molar mass of sulfuric acid
× Molar mass of sulfuric acid
Mass of sulfuric acid =
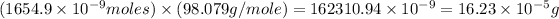
Therefore, the mass of sulfuric acid needed is
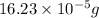 .
.