Answer : The density of a object in cg/L is, 55000 cg/L
Explanation :
As we are given that the density of an object is, 0.55 g/ml.
We have to convert g/ml into cg/L.
As, 1 gram = 100 centigram
As, 1000 milliliter = 1 liter
0.55 g/ml =
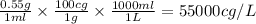
Therefore, the density of a object in cg/L is, 55000 cg/L