Answer : The value of reaction quotient, Q is 0.0625.
Solution : Given,
Concentration of
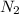 = 2.00 M
= 2.00 M
Concentration of
 = 2.00 M
= 2.00 M
Concentration of
 = 1.00 M
= 1.00 M
Reaction quotient : It is defined as a concentration of a chemical species involved in the chemical reaction.
The balanced equilibrium reaction is,
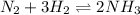
The expression of reaction quotient for this reaction is,
![Q=([Product]^p)/([Reactant]^r)\\Q=([NH_3]^2)/([N_2]^1[H_2]^3)](https://img.qammunity.org/2019/formulas/chemistry/middle-school/dfd9zyxkacmm1qbqpn9tb5xydpwfw0vphz.png)
Now put all the given values in this expression, we get
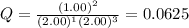
Therefore, the value of reaction quotient, Q is 0.0625.