Answer:- C)
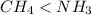 because the NH bond is more polar than CH bond.
because the NH bond is more polar than CH bond.
Explanations:- methane is tetrahedral and it is symmetrical as all the bonds are same. Also, the electron negativity difference of C and H is too low and the molecule is almost non polar and makes the methane to have London dispersion forces.
Ammonia is trigonal pyramidal due to the presence of lone pair of electrons on central nitrogen atom. Nitrogen is highly electron negative atom so the N-H bond is more polar. Also, hydrogen bonding is possible in ammonia as the hydrogen is bonded to more electron negative nitrogen atom.
First option is not correct as the inter molecular forces are weaker in methane as compared to ammonia. Methane has only London dispersion forces where as ammonia has dipole-dipole as well as hydrogen bonding.
Second option is also not correct because the carbon has less partial negative charge as compared to N due to less electron negativity difference of C and H atoms.
Last choice is also not correct since the hydrogen bonding is not present in methane.
So, the only and only correct choice is C)
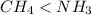 because the NH bond is more polar than CH bond.
because the NH bond is more polar than CH bond.