Answer:
Theoretical yield = 1.85 g
Step-by-step explanation:
Moles of
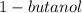 :-
:-
Mass = 1.0 g
Molar mass of
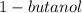 = 74.12 g/mol
= 74.12 g/mol
The formula for the calculation of moles is shown below:
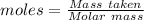
Thus,
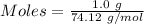
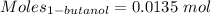
According to the reaction:-
C₄H₉OH + HBr ⇒ C₄H₉Br + H₂O
1 mole of 1-butanol on reaction forms 1 mole of 1-bromobutane
0.0135 mole of 1-butanol on reaction forms 0.0135 mole of 1-bromobutane
Mole of 1-bromobutane = 0.0135 mole
Molar mass of 1-bromobutane = 137.02 g/mol
Mass = Moles * Molar mass = 0.0135 mole * 137.02 g/mol = 1.85 g
Theoretical yield = 1.85 g