Answer : The molecular formula of the compound is
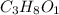 .
.
Solution : Given,
Molecular weight = 60.10 amu
Empirical formula =
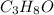
Molar mass of carbon = 12.01 g/mole
Molar mass of hydrogen = 1.01 g/mole
Molar mass of oxygen = 15.99 g/mole
First we have to calculate the Empirical mass of
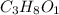 .
.
Empirical mass of
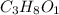 =
=
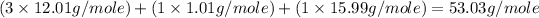
Now we have to calculate the molecular formula.
Divide the molecular weight by the empirical mass and then multiply the subscripts by this number.
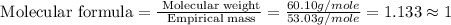
Now multiply the subscripts by 1, we get the molecular formula of the compound.
Therefore, the molecular formula of the compound is
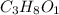 .
.