Hello!
How many molecules of O2 are present in a 3.90L flask at a temperature of 273K and a pressure of 1.00atm?
We have the following data:
V (volume) = 3.90 L
n (number of mols) = ?
T (temperature) = 273 K
P (pressure) = 1 atm
R (gas constant) = 0.082 atm.L / mol.K
We apply the data above to the Clapeyron equation (gas equation), let's see:
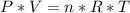
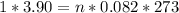
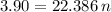
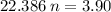
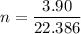
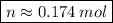
Now, knowing that by Avogadro's Law for each mole of a substance we have 6.022 * 10²³ molecules, how many molecules of O2 are present ?
1 mol -------------------- 6.022*10²³ molecules
0.174 mol ---------------- y molecules
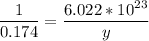
multiply the means by the extremes
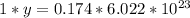

_______________________
I Hope this helps, greetings ... Dexteright02! =)