Answer:
c) RbBr.
Step-by-step explanation:
Hello,
In this case, since rubidium is in group IA, one electron will be eligible to pair during a chemical reaction. In addition, as long as bromine is in group VIIA, when pairing with a metal, just one electron is eligible to pair as well. Now, based on the aforesaid facts, we infer that the undergoing chemical reaction will be:
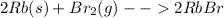
So the formed product is
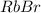 which is rubidium bromide, that is the c) answer.
which is rubidium bromide, that is the c) answer.
Best regards.