According to Charles' law for ideal gases, at constant pressure, the volume of a given mass and moles of gas is directly proportional to its temperature in the Kelvin scale.
Therefore,
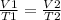
Here,
V1 = Initial volume of decane gas = 23 mL
T1 = Initial temperature of decane gas = 7 degree C = ( 7 + 273.15) K = 280.15 K.
T2 = Final temperature of decane gas = 43 K
V2 = Final volume of decane gas = ?
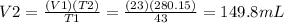
The required volume of decane gas is 149.8 mL.