Answer : The moles of glucose in the sample is
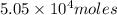 .
.
Solution : Given,
Total number of atoms in a sample of glucose =
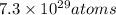
As per the question, 1 mole of glucose molecule contains 6 atom of carbon, 12 atom of hydrogen and 6 atom of oxygen.
Total number of atoms in glucose = 6 + 12 + 6 = 24 atoms
As we know,
1 mole contains
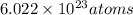
and 1 mole of glucose contains
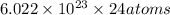
or we can say that,
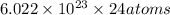 will make 1 mole of glucose molecule
will make 1 mole of glucose molecule
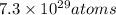 will make
will make
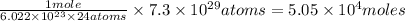 of glucose molecule
of glucose molecule
Therefore, the moles of glucose in the sample is
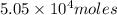 .
.