Answer: Mass of lead in
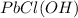 is 107.74 g and mass of lead in
is 107.74 g and mass of lead in
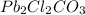 is 103.6 g
is 103.6 g
Step-by-step explanation:
To calculate the number of moles, we use the equation:
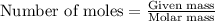 .....(1)
.....(1)
Given mass of PbCl(OH) = 0.135 kg = 135 g (Conversion factor: 1 kg = 1000 g)
Molar mass of PbCl(OH) = 259.66 g/mol
Putting values in equation 1, we get:
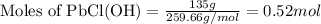
In 1 mole of PbCl(OH), 1 mole of lead, 1 mole of chlorine, 1 mole of hydrogen and 1 mole of oxygen atoms are present.
Moles of lead =
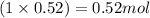
Calculating the mass of lead in PbCl(OH) by using equation 1, we get:
Molar mass of lead atom = 207.2 g/mol
Moles of lead atom = 0.52 moles
Putting values in equation 1, we get:
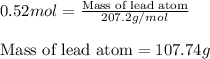
Mass of lead atom = 107.74 g
- For
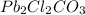 :
:
Given mass of
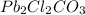 = 0.135 kg = 135 g
= 0.135 kg = 135 g
Molar mass of
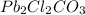 = 545.3 g/mol
= 545.3 g/mol
Putting values in equation 1, we get:
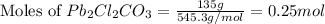
In 1 mole of
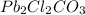 , 2 moles of lead, 2 moles of chlorine, 1 mole of carbon and 3 moles of oxygen atoms are present.
, 2 moles of lead, 2 moles of chlorine, 1 mole of carbon and 3 moles of oxygen atoms are present.
Moles of lead =
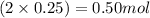
Calculating the mass of lead in
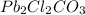 by using equation 1, we get:
by using equation 1, we get:
Molar mass of lead atom = 207.2 g/mol
Moles of lead atom = 0.50 moles
Putting values in equation 1, we get:
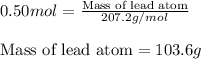
Mass of lead atom = 103.6 g
Hence, mass of lead in
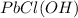 is 107.74 g and mass of lead in
is 107.74 g and mass of lead in
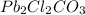 is 103.6 g
is 103.6 g