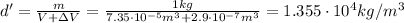a) Density at 100 degrees:
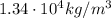
Step-by-step explanation:
The density of mercury at 0 degrees is
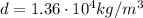
Let's take 1 kg of mercury. Its volume at 0 degrees is
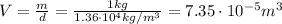
The formula to calculate the volumetric expansion of the mercury is:
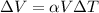
where
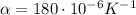 is the cubic expansivity of mercury
is the cubic expansivity of mercury
V is the initial volume
 is the increase in temperature
is the increase in temperature
In this part of the problem,

So, the expansion is
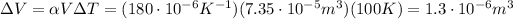
So, the new density is
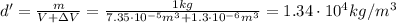
b) Density at 22 degrees:
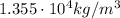
We can apply the same formula we used before, the only difference here is that the increase in temperature is
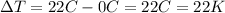
And the volumetric expansion is
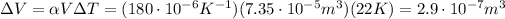
So, the new density is