Answer:
C) Pressure will compress a gas, reducing its volume and giving it a greater density and concentration of particles.
Step-by-step explanation:
At constant temperature, pressure and volume are inversely related.
P V = constant
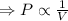
As the pressure increases, the gas compresses, the particles come closer reducing the volume of gas.
As we know, with decrease in volume, density increases.
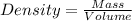
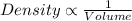
Thus, the pressure of a gas is directly related to concentration of particles. Increase in pressure causes increase in concentration of the particles.