Answer : The products are Silver sulfide,
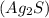 and Sodium iodide,
and Sodium iodide,
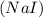 .
.
Explanation :
The given balanced chemical reaction is,
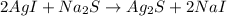
From the given balanced reaction, we conclude that the 2 moles of silver iodide react with the 1 mole of sodium sulfide to give product as 1 mole of silver sulfide and 2 moles of sodium iodide.
In a chemical reaction, reactants are represent on the left side of the right-arrow and products are represent on the right side of the right-arrow.
Therefore, in a chemical reaction the products are Silver sulfide and Sodium iodide.