Answer: Energy required for the ionization of mercury is

Explanation: Energy of the electron in a hydrogen-like ion is given by the equation:
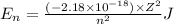
where, Z = atomic number
n = principle quantum number
Ionization energy is the energy required to release the outermost electron from an isolated gaseous atom.
For the ionization of mercury, the equation follows:
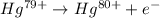
Mercury has an atomic number = 80
As, in this element 79 protons are already released, which means that the electronic configuration for
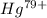 is
is
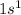
and the principle quantum number for the last ionization step = 1
Putting the values in energy equation, we get
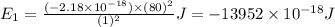
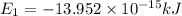 (Conversion Factor: 1kJ = 1000J)
(Conversion Factor: 1kJ = 1000J)