Answer: Option (A) is the correct answer.
Step-by-step explanation:
A balanced equation is defined as the equation where number of atoms of the reactant equal to the number of atoms of the product.
This also means that mass of the reactants is equal to the mass of products in a chemical equation.
For example,
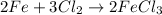
But when the number of moles of reactants is not equal to the number of moles of products then the reaction equation is not balanced.
For example,
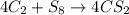
Here, the moles of carbon atom are not equal on both reactant and product side. Hence, this reaction equation is not balanced.
Thus, we can conclude that out of the given options
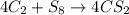 , this chemical reaction model is flawed.
, this chemical reaction model is flawed.