Start by assigning the most complicated species a coefficient of one. That species should contain the greatest number of elements, e.g.
 .
.
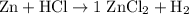 (not balanced)
(not balanced)
Assign coefficients to the rest of the species based on the conservation of atoms. For instance, the left hand side of the equation now contains one atom of hydrogen H and one atoms of chlorine Cl. The left hand side shall have an identical configuration. Both zinc chloride
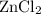 and hydrogen
and hydrogen
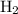 should therefore have a coefficient of 1/2. (Don't panic about the fractions. They are to be eliminated in a few more steps.)
should therefore have a coefficient of 1/2. (Don't panic about the fractions. They are to be eliminated in a few more steps.)
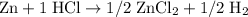 (not balanced)
(not balanced)
The coefficient 1/2 in front of zinc chloride indicates the presence of 1/2 zinc atom in the right hand side of the equation. Zinc on the left hand side of the equation should accordingly have a coefficient of 1/2.
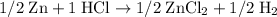
Increasing coefficients on both sides of the equation by a factor of two to eliminate all fractions. Hence the balanced equation.
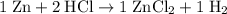 (balanced)
(balanced)
The same set of operations should work for the second equation.
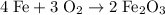 (balanced)
(balanced)
Note that the third equation does not accurately represent the catalytic decomposition of hydrogen peroxide
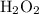 . The balanced equation should be:
. The balanced equation should be:
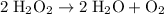 (balanced)
(balanced)