Answer:
D. Their valence electrons can be in both s and f sublevels.
Explanation:
The inner transition elements are those in the two long rows at the bottom of the Periodic Table.
The lanthanide series starts after Ba in Period 6, and the actinide series starts after Ra in Period 7.
Thus, we would predict their electron configurations to be of the form
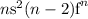
However, the energy levels of the ns, (n-1)d, and (n-2)f orbitals are so close in energy that there are many exceptions to our predictions
For example, here are some electron configurations.
La = [Xe]6s²5d (not [Xe]6s²4f)
Ce = [Xe]6s²4f5d (not [Xe]6s²4f²)
Pr = [Xe]6s²4f³ (as predicted)
Thus, their valence electrons can be in both s and f (and sometimes d) sublevels.
A. Wrong. The inner transition elements do not include the elements in Groups 3 to 12. They are the elements between Groups 2 and 3.
B. Wrong. They do not occupy the d block (those are the transition metals). They occupy the f block.
C. Wrong. They include the lanthanides and actinides, but most of them have at least one electron in an f sublevel.