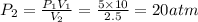We assume the change in volume occurs at constant temperature and so we use the Boyle's law formula
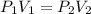 .
.
We are given the initial volume and pressure as well as as final volume, the question wants the final pressure
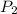
So we make
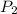 the subject of the formula
the subject of the formula