Answer:
630 mmHg is the required new pressure.
Step-by-step explanation:
At constant temperature;
Initial volume of the gas,
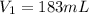
Initial pressure exerted by the gas,
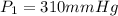
Final volume of the gas ,
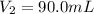
Final pressure exerted by the gas ,
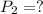
At constant temperature pressure and volume of the gas varies indirectly.
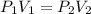 (Boyle's Law)
(Boyle's Law)
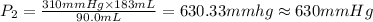
630 mmHg is the required new pressure.