Answer :
(a) The maximum number of electrons in
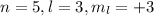 are, 2 electrons.
are, 2 electrons.
(b) The maximum number of electrons in
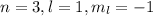 are, 2 electrons.
are, 2 electrons.
Explanation :
Azimuthal Quantum Number : It describes the shape of the orbital. It is represented as 'l'. The value of l ranges from 0 to (n-1). For l = 0,1,2,3... the orbitals are s, p, d, f...
Magnetic Quantum Number : It describes the orientation of the orbitals. It is represented as
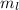 . The value of this quantum number ranges from
. The value of this quantum number ranges from
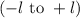 . When l = 2, the value of
. When l = 2, the value of
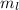 will be -2, -1, 0, +1, +2.
will be -2, -1, 0, +1, +2.
Spin Quantum number : It describes the direction of electron spin. This is represented as
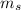 The value of this is
The value of this is
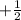 for upward spin and
for upward spin and
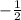 for downward spin.
for downward spin.
(a)
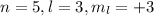
As per above information,
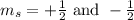 (For each sub-shell)
(For each sub-shell)
From this we conclude that,
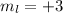 means there are 1 orbital and each orbital contains 2 electrons. So, the number of electrons held in this will be, 2 electrons
means there are 1 orbital and each orbital contains 2 electrons. So, the number of electrons held in this will be, 2 electrons
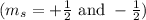 .
.
(b)
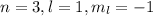
As per above information,
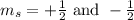 (For each sub-shell)
(For each sub-shell)
From this we conclude that,
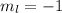 means there are 1 orbital and each orbital contains 2 electrons. So, the number of electrons held in this will be, 2 electrons
means there are 1 orbital and each orbital contains 2 electrons. So, the number of electrons held in this will be, 2 electrons
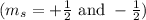 .
.