Answer:- 768 J.
Solution:- 2.30 g of ice is present at water's melting point that is 0 degree C. Heat of fusion is given as 334 J per g and we are asked to calculate the energy absorbed to melt the ice.
334 J per g means 334 J of heat is required to melt one gram of ice. How much of heat would be required to melt 2.30 g of ice.
the equation used is,
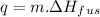
where q is heat energy, m is mass and
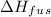 is the enthalpy of fusion.
is the enthalpy of fusion.
Let's plug in the values:
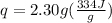
q = 768.2 J
If rounded for correct number of sig figs then it is 768 J. So, 768 J of energy is absorbed to melt 2.30 g of ice.