Here we have to calculate the number of moles of valuable propane can be prepared from 1.8 moles of carbon.
From 1.8 moles of carbon 0.3 moles of propane can be prepared by the reaction.
From 6 moles of carbon (C) 1 moles of valuable propane (C₃H₈) can be prepared.
Thus from 1.8 moles of C we can obtain
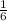 ×1.8 = 0.3 moles of the propane can be prepared.
×1.8 = 0.3 moles of the propane can be prepared.
Thus the amount of propane produced in this reaction is determined.