Answer:
Pressure = 0.62 atm
Step-by-step explanation:
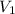 = 85
= 85
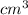 ,
,
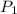 = 1.45 atm,
= 1.45 atm,
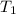 = 310 K,
= 310 K,
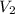 = 180
= 180
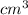 ,
,
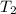 = 280 K.
= 280 K.
Applying the general gas law,
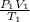 =
=
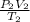
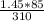 =
=
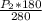
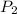 =
=
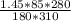
=
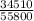
= 0.62
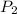 = 0.62 atm
= 0.62 atm
The pressure when the volume is increased and the temperature reduced to the given values is 0.62 atm.