Answer: The formula of the complex will be
![[Fe(NH_2CH_2CH_2NH_2)_3]^(3+)\text{ or }[Fe(en)_3]^(3+)](https://img.qammunity.org/2019/formulas/chemistry/college/dlrmv7njfqf1v7dblkvrqlybnxie0wofmx.png)
Step-by-step explanation:
Coordination number in a complex is defined as the number of ligands that are attached to the central metal atom.
- If the ligand is unidentate, it will occupy 1 place around the central metal atom.
- If the ligand is bidentate, it will occupy 2 places around the central metal atom.
- If the ligand is tridentate, it will occupy 3 places around the central metal atom.
We are given:
A metal ion having formula
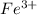 and ligand attached to it is ethylenediamine (shortly named as 'en')
and ligand attached to it is ethylenediamine (shortly named as 'en')
Coordination number of the complex is 6
We know that, 'en' is a bidentate ligand and does not carry any charge on it
Hence, the formula of the complex will be
![[Fe(NH_2CH_2CH_2NH_2)_3]^(3+)\text{ or }[Fe(en)_3]^(3+)](https://img.qammunity.org/2019/formulas/chemistry/college/dlrmv7njfqf1v7dblkvrqlybnxie0wofmx.png)