Strong acids can dissolve the salts of weak acid. When we consider the different salts of silver:
Salts of silver with the conjugate bases of a weak acid are soluble in strong acidic solutions. Some of these salts are:
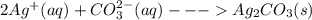
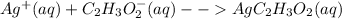
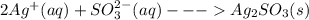
Salts of silver with the conjugate bases of a strong acid are not affected by change in pH:
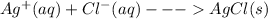
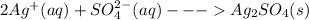
These two salts with Chloride and sulfate ions are not soluble in acidic solutions as the salts of silver with the conjugate bases of a strong acid are not soluble in acidic solutions, they remain unaffected by any change in pH.
So for salts of Ag and Ba with the conjugate bases of a weak acid, solubility is increased upon the addition of an acid. So, the interference from the ions of weak acids can be removed by decreasing the pH.