Answer : The strength of drysol is 178.55 g/L.
Solution : Given,
Mass of aluminium chloride hexahydrate = 15 g
Volume of alcohol = 84 ml
Molar mass of Drysol = 241.422 g/mole
First we have to calculate the concentration of drysol.
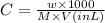
where,
C = concentration of drysol
w = Mass of drysol
M = molar mass of drysol
V = Volume of drysol
Now put all the given values in above formula,we get
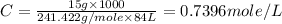
Now we have to calculate the strength of drysol.
Strength of drysol = Concentration of drysol × Molar mass of drysol
Strength of drysol = 0.7396 mole/L × 241.422 g/mole = 178.55 g/L
Therefore, the strength of drysol is 178.55 g/L.