Answer : The correct option is A.
Explanation :
Law of conservation of mass : In the chemical reaction, the mass of reactant must be equal to the mass of product.
A.
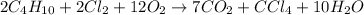
The mass of reactant side =
![2C_4H_(10)+2Cl_2+12O_2=[8(12)+20(1)]+4(35.5)+24(16)=642g](https://img.qammunity.org/2019/formulas/chemistry/middle-school/qcvhm54g2ir0ggl87dz0kez8l00rduhao6.png)
The mass of product side =
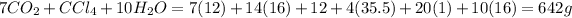
This means, the mass of product is equal to the mass of reactant. The mass remains conserved and obeys the law of conservation of mass.
The reaction B, C, D, E does not obey the law of conservation of mass.
Therefore, Only reaction A obey the law of conservation of mass.