Answer:- 16100 mg HCN
Solution:- From the given dimensions of the room we calculate it's volume and then do the unit conversion from cubit feet to cubic centimeter as the density is given in grams per cubic centimeter. Then the volume is multiplied with the density to get the mass of air and finally this is multiplied by the lethal dose to calculate the amount of HCN required.
Volume of room =
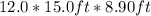 =
=
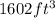
Unit conversion from cubic feet to cubic centimeter:
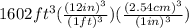
=
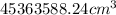
(For above unit conversion what we have used is, 1ft = 12in and 1in = 2.54cm)
mass of air = volume x density
=
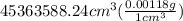
= 53529.03 g
amount of HCN = mass of air x lethal dose
amount of HCN =
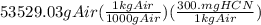
= 16058.71 mg HCN
So, 16058.71 mg of HCN will give the lethal dose in the laboratory room.
If we report the answer with three sig figs then it could be round to 16100 mg.