Answer:
All the given substances are insoluble in water
Step-by-step explanation:
According to solubility rule-
(1) All sulfides are insoluble except alkali and alkaline earth metals.
So, ZnS is insoluble in water.
(2) All carbonates are insoluble except
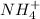 and alkali metals.
and alkali metals.
So,
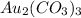 is insoluble in water.
is insoluble in water.
(3) All chlorides are soluble except AgCl,
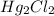 and
and
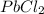
So,
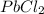 is insoluble in water.
is insoluble in water.
(4) All metallic oxides are insoluble except
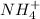 and alkali metals.
and alkali metals.
So,
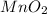 is insoluble in water.
is insoluble in water.